EPN successfully held a webinar on: ”Innovative Solutions for Quality Assurance: Leveraging the capabilities of Minilab Standard and Falsified Detection” Webinar on Thursday 22 February, 2024.
Over 100 participants from 37 Francophone, Lusophone and Anglophone countries including National Regulatory Authorities, National Quality Control Laboratories and Food and Drug Administration representatives participated in the Webinar.
There was exchange of knowledge from partners like PQM+, GPHF, academic institutions represented by Professor Lutz Heide from the University of Tuebingen and Difaem followed by a panel discussion of experience sharing from EPN minilab partners in Cameroon (PCC), Malawi (CHAM) and Nigeria (CHAN-MEDIPHARM).
Strengthening Regulatory and Quality Control systems to ensure quality medical products
Presented by Dr. Zelalem Mamo, Chief of Party, USP PQM+, Ethiopia.
 USP PQM+ emphasised on the significance of strengthening regulatory and quality control systems, networking and collaboration with national/regional regulatory agencies in the countries and globally. It was noted that only 5 African countries have reached the Maturity Level 3 (Level 5 being the highest). These are Egypt and South Africa ML 3 for vaccines (producing), Tanzania and Ghana for medicines and vaccines (nonproducing) and Nigeria for medicines (producing). PQM+ showcased the role of the two testing levels of the cost-effective Minilab: the visual/physical inspection (level 1) and the semi-quantitative Thin Layer Chromatography (level 2).
USP PQM+ emphasised on the significance of strengthening regulatory and quality control systems, networking and collaboration with national/regional regulatory agencies in the countries and globally. It was noted that only 5 African countries have reached the Maturity Level 3 (Level 5 being the highest). These are Egypt and South Africa ML 3 for vaccines (producing), Tanzania and Ghana for medicines and vaccines (nonproducing) and Nigeria for medicines (producing). PQM+ showcased the role of the two testing levels of the cost-effective Minilab: the visual/physical inspection (level 1) and the semi-quantitative Thin Layer Chromatography (level 2).
EPN/Difaem Minilab Network work with the member MEDS for the Compendial testing (Level 3) which is the most complex, expensive and time-consuming type of testing. University of Tubingen support also our work with level 3 testing in research perspective.
Using a risk-based approach in sample collection and testing can reduce costs and ensure that the right products have been sampled for routine monitoring of quality.
The challenge of substandard and falsified medicines in the church health system in Nigeria and lessons learned
A recent study with FBCMF – presented by Prof. Lutz Heide.
In this study, 260 medicine samples were collected, containing 13 Active Pharmaceutical Ingredients (API). Two sources were targeted, “Licensed vendors” from a list of 74 manufacturers/wholesalers and the “Pharmaceutical markets” in Onitsha & Enugu with unclear licensing status. The results of the study indicate that 1.5% of tested samples were falsified and 23.9% substandard. It was also found similar proportion of substandard medicines in licensed vendors and in unregulated markets.
Lessons learned:
EPN/Difaem Minilab Network testing Jan – Dec 2023
The report shared by Dr Gabriela Gohl, Pharmacist from Difaem on the Minilab Network revealed that;
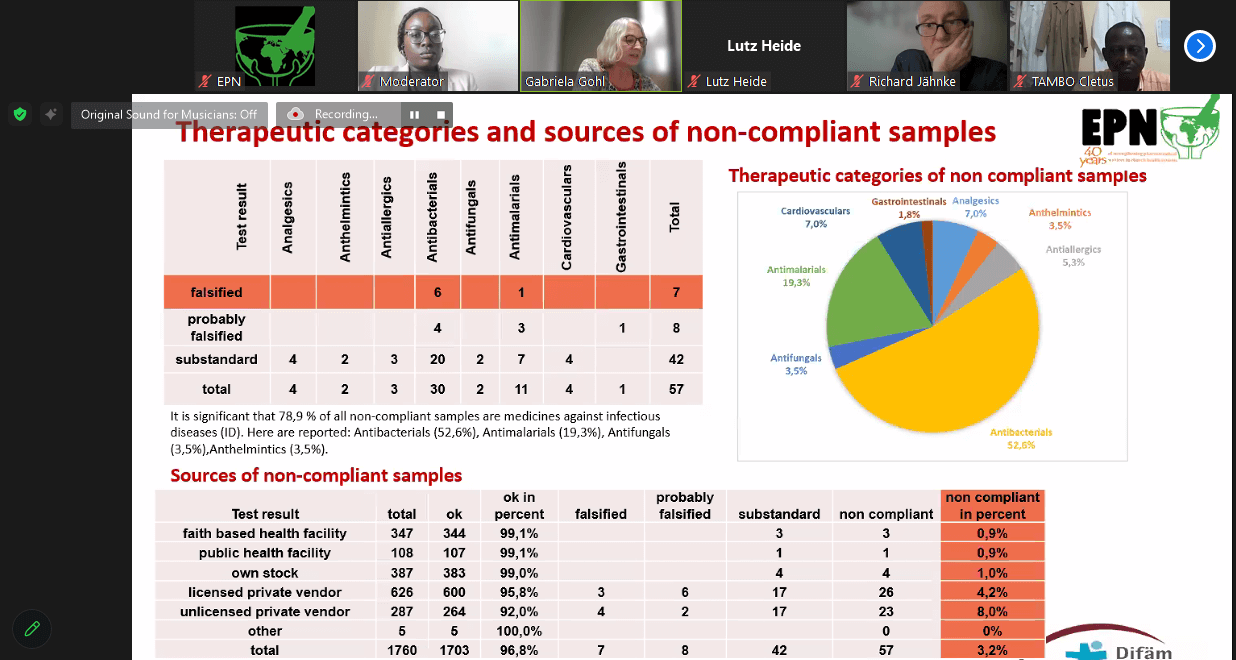
There in a need to continue monitoring the quality of antibiotics in the EPN approach of combating AMR in the church health system. In this perspective, the GPHF Minilab is a powerful tool in addressing the raising threat of AMR.
Members FBCMF and CHAN Nigeria, CHAM Malawi, PCC and CBC Cameroon shared their experience in using the Minilab, its impact in DSO’s quality assurance system and the need to improve procurement of medicine practices in their pharmaceutical supply chain systems. Risk-based sampling of medicine including building capacities of the Minilab users and reporting to the World Health Organization (WHO) are of importance to EPN members.
Conclusion
Curbing the proliferation of unsafe medicine and preservation of human life and through ensuring provision of safe medicines for all communities regardless of their status is essential as a basic human right. The webinar concluded with a call for improvements in medicine procurement practices and sustained multi-actor collaboration to enhance medicines quality control measures.
EPN shall continue to support all efforts to fight substandard and falsified medicines in members health facilities especially in the African region.
 We thank our speakers and partners Dr. Richard Jähnke, Project manager GPHF, Germany, Dr. Zelalem Mamo, Chief of Party, USP PQM+, Ethiopia, Prof. Lutz Heide, Professor of Pharmaceutical, Biology, University of Tübingen, Germany, Ms. Gabriela Gohl, Pharmaceutical projects and procurement, Difaem, Germany and members Rev. Sr. Jane Frances, Chief Executive Officer, Faith Based Central Medical Foundation (FBCMF), Nigeria, Evans Chirambo, Pharmacy Manager, Christian Health Association of Malawi (CHAM), Dr. Edmund NEBA, Presbyterian Church In Cameroon (PCC) and Ms. Adetunji Temitope, Pharmacist/ Head Minilab, CHAN Medi-Pharm, Nigeria for their valuable insights during the webinar
We thank our speakers and partners Dr. Richard Jähnke, Project manager GPHF, Germany, Dr. Zelalem Mamo, Chief of Party, USP PQM+, Ethiopia, Prof. Lutz Heide, Professor of Pharmaceutical, Biology, University of Tübingen, Germany, Ms. Gabriela Gohl, Pharmaceutical projects and procurement, Difaem, Germany and members Rev. Sr. Jane Frances, Chief Executive Officer, Faith Based Central Medical Foundation (FBCMF), Nigeria, Evans Chirambo, Pharmacy Manager, Christian Health Association of Malawi (CHAM), Dr. Edmund NEBA, Presbyterian Church In Cameroon (PCC) and Ms. Adetunji Temitope, Pharmacist/ Head Minilab, CHAN Medi-Pharm, Nigeria for their valuable insights during the webinar
IMPORTANT INFORMATION TO NOTE!
Existing users of the Minilab are invited to send their suggestions and ideas on how the Minilab can become even better in the future, for example new testing protocols, new compounds to be tested, new technical features, new training tools and beyond.
Please send your suggestions and ideas to info@epnetwork.org and communication@epnetwork.org.
Webinar video recording